Abstract
Background: Current methods to evaluate therapeutic response in Acute Myeloid leukemia (AML) rely primarily on morphological evaluation of leukemia blasts in bone marrow at end of treatment cycles, with complete remission (CR) defined as less than 5% bone marrow blast counts without leukemic phenotype. Measurable residual disease (MRD) has been shown highly prognostic for outcome. MRD by molecular methods is limited to subgroup of patients with defined genetic mutations. MRD detection by Multicolor Flow Cytometry (MFC) is still being standardized. Leukemia stem cells (LSC) has been established as source of treatment resistance and disease recurrence in AML. In this study, we aim to analyze change in LSC during treatment using immunophenotypically defined markers and examine whether information gained from LSC analysis could provide additional insights into disease course and assist management of AML under routine clinical practice setting.
Methods: We analyzed flow cytometry data from bone marrow aspirate (BMA) and/or peripheral blood (PB) in patients with AML (excluding acute promyelocytic leukemia) before and after treatment. "Leukemic blasts” were identified by morphology and Leukemia-associated Immunophenotype by MFC. Hematopoietic stem cells (HSCs) gating was based on CD45dim/SSC and CD34+CD38low/- expression. Leukemic stem cells (LSC) fractional change was analyzed using CD371 (CLL-1), CD366 (TIM3), and CD45RA percent of positive over HSCs. A chart review was conducted to determine correlation between LSC fractional change and clinical outcome. Statistical analysis was performed using GraphPad Prism version 9.
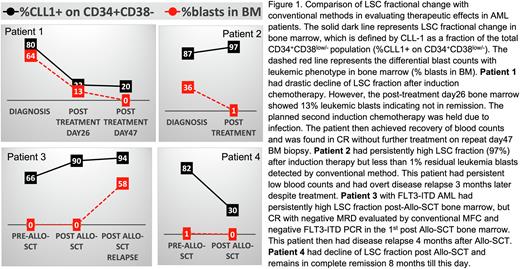
Results: Fifty AML cases with both pre- and post-treatment data from a single institution in the past 3 years were included in this study. Consistent with published literature, patients with significant reduction in LSC subsets was associated with longer remission, while patients with no significant decrease in the LSC subsets demonstrated no or only a temporary remission. Intriguingly, several patients with drastic reduction in LSC after induction chemotherapy achieved complete remission, despite initial post-treatment bone marrow showing persistence of ">5% leukemia blasts” indicating possibly failed induction therapy. In contrast, in patients with persistently high or increased LSC fraction post-treatment, overt clinical relapse occurred quickly despite "absence” or <1% of leukemia blast differential counts in initial bone marrow. In a series of patients who received allogeneic stem cell transplant (Allo-SCT), rise of LSC fraction post Allo-SCT was associated with early AML relapse (within 6 months), while the decrease of LSC fraction was associated with prolonged remission (Representative patient cases shown in Figure 1). Lastly, among the three LSC-specific markers analyzed, the changes in LSC characterized by CLL-1 expression showed the best correlation with clinical course. In addition, the LSC fraction measured by CLL-1 in the peripheral blood is highly correlated with that of the bone marrow (R2=0.93, P<0.0001).
Conclusion: This retrospective study explored the value and applicability of LSC detection in routine clinical practice. Importantly, conventional methods relying on evaluating leukemic blast counts in post-treatment bone marrow can sometimes over- or under- estimate the benefit of certain treatment regimen, leading to inappropriate continuation of the same treatment or switch of a potentially effective treatment regimen. In those groups of patients, the change in LSC fraction provides additional information for early treatment response evaluation, refines outcome prediction, and helps with treatment regimen selection. Trending LSC fractional change identifies impending relapse in post Allo-SCT. LSC fractional change determined by CLL-1 showed the best clinical correlation and should be incorporated into routine evaluation for AML patient in clinical practice, and possibly for those participating in investigational trials. Future studies will focus on collecting more data to validate above findings, determining the thresholds of LSC fractional change which constitute potential treatment response vs failure and testing the feasibility of tracking LSC subsets non-invasively in peripheral blood during treatment to predict therapeutic outcome and potentially guide treatment decisions.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal